Types of Drug Eluting Stents
This is the third part of our series on Drug Eluting Stents. We already covered what DES are. In part three we discuss the type of stents.
As they say "imitation is the best form of flattery". The first DES was released in 2002. Since this initial release a whole host of DES is now available. Even categorizing them is difficult. Some of the DES being sold have almost no clinical data and so their use is entirely at the patients’ and doctor’s own discretion and responsibility. Allow me to introduce the way I categorize the available DES. These are wholly my thoughts so comments are welcome.
Category 1
These are patented stents manufactured by companies who have done larges-scale clinical trials. This means safer and more effective product. They are approved by certifying bodies. Since Malaysia has yet to have such a body then typically we depend on the FDA or the CE mark. These typically cost RM 6,500 - RM 8,000 (USD 1800 to 2000). In Malaysia you could probably get Cypher (JnJ Cordis), Taxus (Boston Scientific) or Endeavour (AVE Medtronic).
Category 2
These are usually patented stents which are undergoing clinical trials. Their safety and effectiveness is not yet well established. These are typically costly. Locally we are looking at XienceV (Guidant) and Crura (Niche Medical)
Category 3
These stents are manufactured by small companies, with little background information, both on the companies, and the stents. Quality control is obviously lacking here, and post market surveillance is lacking. The advantage is that they are cheaper costing between RM 5,000 and RM 6,000. Another way of categorizing DES types is by mechanism of action of the drugs involved. The most popular DES in USA at the moment seem to be the Cypher DES manufactured by JnJ cordis. The active drug there is sirolimus, a drug that was first use in to lessen rejection in kidney transplantation. It essentially work by stopping cell multiplication (is cytostatic). It is the first DES to obtain FDA approval, and so far has proven very effective and safe, both locally and worldwide.
Another way of categorizing DES types is by mechanism of action of the drugs involved. The most popular DES in USA at the moment seem to be the Cypher DES manufactured by JnJ cordis. The active drug there is sirolimus, a drug that was first use in to lessen rejection in kidney transplantation. It essentially work by stopping cell multiplication (is cytostatic). It is the first DES to obtain FDA approval, and so far has proven very effective and safe, both locally and worldwide.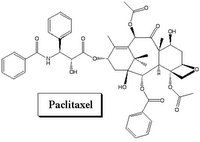
The second stent to receive FDA approval is the Taxus stent, and the drug belong to a different class, called paclitaxel. This agent is sometimes used in treatment of breast cancer. It acts by killing cells (cytotoxic) to prevent them from multiplying. It has also been proven to be safe and effective.
Following these two, currently with CE mark approval, and awaiting FDA approval is the Endevour stent (by AVE Medtronic) and the XienceV stent (by Guidant). Both these stents have compounds almost similar to sirolimus. We call them the –Olimus sisters. They act primarily to halt cell multiplication. In the case of Endevour, the drug is Zitarolimus, and in the case of XienceV, the drug is Everolimus. The Endevour stent is currently available in Malaysia, while the XienceV is yet to arrive. So far, the medical data on the Cypher, Taxus and Endevour stent has been good.
No comments:
Post a Comment